4.9 KiB
Executable File
author, date, title, tags
| author | date | title | tags | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Alvie Rahman | \today | MMME1048 // Fluid Mechanics |
|
Lecture 1 // Properties of Fluids (2021-10-06)
What is a Fluid?
- A fluid may be liquid, vapor, or gas
- No permanent shape
- Consists of atoms in random motion and continual collision
- Easy to deform
- Liquids have fixed volume, gasses fill up container
- A fluid is a substance for wich a shear stress tends to produce unlimited, continuous deformation
Shear Forces
- For a solid, application of shear stress causes a deformation which, if not too great (elastic), is not permanent and solid regains original positon
- For a fluid, continuious deformation takes place as the molecules slide over each other until the force is removed
- A fluid is a substance for wich a shear stress tends to produce unlimited, continuous deformation
Density
- Density:
\rho = \frac m V - Specific Density:
v = \frac 1 \rho
Obtaining Density
-
Find mass of a given volume or volume of a given mass
-
This gives average density and assumes density is the same throughout
- This is not always the case (like in chocolate chip ice cream)
- Bulk density is often used to refer to average density
Engineering Density
- Matter is not continuous on molecular scale
- For fluids in constant motion, we take a time average
- For most practical purposes, matter is considered to be homogenous and time averaged
Pressure
-
Pressure is a scalar quantity
-
Gases cannot sustain tensile stress, liquids a negligible amount
-
There is a certain amount of energy associated with the random continuous motion of the molecules
-
Higher pressure fluids tend to have more energy in their molecules
How Does Molecular Motion Create Force?
-
When molecules interact with each other, there is no net force
-
When they interact with walls, there is a resultant force perpendicular to the surface
-
Pressure caused my molecule:
p = \frac {\delta{}F}{\delta{}A} -
If we want total force, we have to add them all up
-
F = \int \mathrm{d}F = \int p\, \mathrm{d}A- If pressure is constant, then this integrates to
F = pA - These equations can be used if pressure is constant of average value is appropriate
- For many cases in fluids pressure is not constant
- If pressure is constant, then this integrates to
Pressure Variation in a Static Fluid
- A fluid at rest has constant pressure horizontally
- That's why liquid surfaces are flat
- But fluids at rest do have a vertical gradient, where lower parts have higher presure
How Does Pressure Vary with Depth?
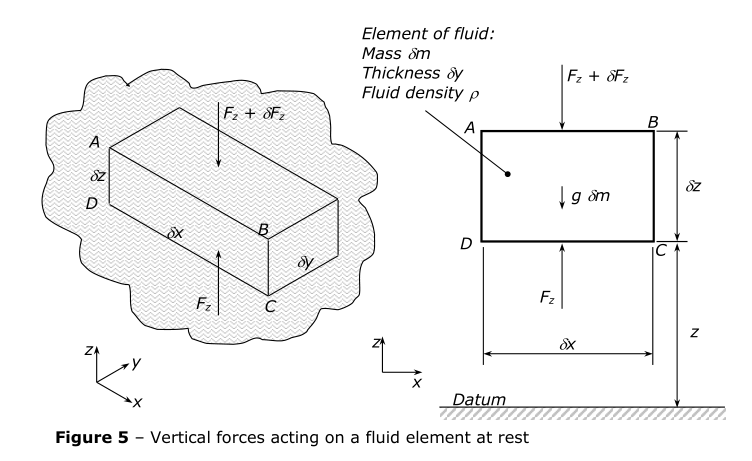
Let fluid pressure be p at height z, and p + \delta p at z + \delta z.
Force F_z acts upwards to support the fluid, countering pressure p.
Force $F_z + \delta F_z$acts downwards to counter pressure p + \delta p and comes from the weight
of the liquid above.
Now:
\begin{align*} F_z &= p\delta x\delta y \ F_z + \delta F_z &= (p + \delta p) \delta x \delta y \ \therefore \delta F_z &= \delta p(\delta x\delta y) \end{align*}
Resolving forces in z direction:
\begin{align*} F_z - (F_z + \delta F_z) - g\delta m &= 0 \ \text{but } \delta m &= \rho\delta x\delta y\delta z \ \therefore -\delta p(\delta x\delta y) &= g\rho(\delta x\delta y\delta z) \ \text{or } \frac{\delta p}{\delta z} &= -\rho g \ \text{as } \delta z \rightarrow 0,, \frac{\delta p}{\delta z} &\rightarrow \frac{dp}{dz}\ \therefore \frac{dp}{dz} &= -\rho g\ \Delta p &= \rho g\Delta z \end{align*}
The equation applies for any fluid.
The -ve sign indicates that as z, height, increases, p, pressure, decreases.
Absolute and Gauge Pressure
-
Absolute Pressure is measured relative to zero (a vacuum)
-
Guage pressure = absolute pressure - atmospheric pressure
- Often used in industry
-
If abs. pressure = 3 bar and atmospheric pressure is 1 bar, then gauge pressure = 2 bar
-
Atmospheric pressure changes with altitude
Compressibility
- All fluids are compressible, especially gasses
- Most liquids can be considered incompressible most of the time (and will be in MMME1048, but may not be in future modules)
Surface Tension
- In a liquid, molecules are held together by molecular attraction
- At a boundry between two fluids this creates "surface tension"
- Surface tension usually has the symbol
\gamma
Ideal Gas
-
No real gas is perfect, although many are similar
-
We define a specific gas constant to allow us to analyse the behaviour of a specific gas:
R = \frac {\tilde R}{\tilde m}(Universal Gas Constant / molar mass of gas)
-
Perfect gas law
pV=mRTor
p = \rho RT- Pressure always in Pa
- Temperature always in K
Units and Dimentional Analysis
- It is usually better to use SI units
- If in doubt, DA can be useful to check that your answer makes sense