16 KiB
Executable File
| author | date | title | tags | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Alvie Rahman | \today | MMME1029 // Materials |
|
\tableofcontents
Lecture 1 (2021-10-04)
1A Reading Notes
Classification of Energy-Related Materials
-
Passive materials---do not take part in energy conversion e.g. structures in pipelines, turbine blades, oil drills
-
Active materials---directly take part in energy conversion e.g. solar cells, batteries, catalysts, superconducting magnests
-
The material and chemical problems for conventional energy systems are mostly well understood and usually associated wit structural and mechanical properties or long standing chemical effects like corrosion:
- fossil fuels
- hydroelectric
- oil from shale and tar
- sands
- coal gasification
- liquefaction
- geothermal energy
- wind power
- bomass conversion
- solar cells
- nuclear reactors
Applications of Energy-Related Materials
High Temperature Materials (and Theoretical Thermodynamic Efficiency)
-
Thermodynamics indicated that the higher the temperature, the greater the efficiency of heat to work:
\frac{ T_{high} - T_{low} }{ T_{high} } -
The first steam engines were only 1% efficient, while modern steam engines are 35% efficient primarily due to improved high-temperature materials.
-
Early engines made from cast iron while modern engines made from alloys containing nickel, molybdenum, chromium, and silicon, which don't fail at temperature above 540 \textdegree{}C
-
Modern combustion engines are nearing the limits of metals so new materials that can function at even higher temperatures must be found--- particularly intermetallic compounds and ceramics are being developed
Types of Stainless Steel
- Type 304---common; iron, carbon, nickel, and chromium
- Type 316---expensive; iron, carbon, chromium, nickel, molybdenum
Self Quiz 1
-
What is made of billion year old carbon + water + sprinkling of stardust?
Me
-
What are the main classifications of materials?
Metals, glass and ceramics,
plastics, elastomers,polymers, composites, and semiconductors -
[There are] Few Iron Age artefacts left. Why?
They rusted away
-
What is maens by 'the micro-structure of a material'?
The very small scale structure of a material which can have strong influence on its physical properties like toughness and ductility and corrosion resistance
-
What is a 'micrograph' of a material?
A picture taken through a microscope
-
What microscope is used to investage the microstructure of a material down to a 1 micron scale resolution?
Optical Microscope
-
What microscope is used [to investigate] the microstructure of a material down to a 100 nm scale resolution?
Scanning Electron Microscope
-
What length scales did you see in the first slide set?
1 mm, 0.5 mm, 1.5 \textmu{}m
-
What material properties were mentioned in the first slide set?
Hardness, brittleness, melting point, corrosion, density, thermal insulation
Self Quiz 2
-
What is the effect of lowering the temperature of rubber?
Makes it more brittle, much less elastic and flexible
-
What material properties were mentioned in the second slide set?
Young's modulus, specific heat, coefficient of thermal expansion
Lecture 2
Properties of the Classes
Metals
- Ductile (yields before fracture)
- High UFS (Ultimate Fracture Stress) in tension and compression
- Hard
- Tough
- High melting point
- High electric and thermal conductivity
Ceramics and Glasses
- Brittle --- elastic to failure, no yield
- Hard (harder than metals)
- Low UFS under tension
- High UFS under compression
- Not tough
- High melting points
- Do not burn as oxide ceramics are already oxides
- Chemically resistant
- Poor thermal and electric conductivity
- Wide range of magnetic and dielectric behaviours
Polymers
-
Organic---as in organic chemistry (i.e. carbon based)
-
Ductile
-
Low UFS in tension and compression
-
Not hard
-
Reasonably tough
-
Low threshold temperature to charring and combustion in air or pure oxygen
-
Low electrical and thermal conductivity
- There are some electrically conductive polymers
Composites
-
Composed of 2 or more materials on any scale from atomic to mm scale to produce properties that cannot be obtained in a single material
- Larger scale mixes of materials may be called 'multimaterial'
-
Material propertes depends on what its made of
Terms
Organic vs Inorganic Materials
- Organic materials are carbon based
- From chemistry, organic compounds are ones with a C-H bond
- Inorganic compounds do not contain the C-H bond
Crystalline vs Non-Crystalline Materials
-
Most things are crystalline
- Ice
- Sugar
- Salt
- Metals
- Ceramics
-
Glasses are non-crystalline
Material Properties
Density
\rho = \frac m v-
Density if quoted at STP (standard temperature and pressure---
298K and1.013\times 10^5Pa) -
Metals, ceramics, and glasses are high density materials
-
Polymers are low density
-
Composites span a wide range of density as it depends on the materials it is composed of
e.g. composites with a metal matrix will have a much higher density than those with a polymer matrix
Melting Points
-
Measured at standard pressure and in an intert atmosphere (e.g. with Nitrogen, Argon, etc)
- Diamond and graphite will survive up to 4000 \textdegree{}C in an inert atmosphere but would burn at around 1000 \textdegree{}C in oxygen
-
High melting points -> high chemical bond strength
Corrosion
-
It's not just metals that corrode
-
Polymers
- UV degradation
- Water absorption can occur in degraded polymers
-
Glass
- Leaching
- Sodium ions can leave the glass when covered in water. If the water stays, the high pH water can damage the class
Self Quiz
Consolidation Questions 1
i. Metal ii. Titanium
-
i. Polymer ii. Polyester
i. Ceramic ii. Alumino-silicate
i. Composite ii. GFRP or CFRP
i. Metal ii. Aluiminium alloy
i. Metal ii. Aluiminium alloy
i. Polymer ii. Acrylic
i. Ceramimc ii. Glass
i. Composite ii. Concrete
Consolidation Questions 2
CB
Polymers
Introduction to Polymers
There are 3 types of polymers:
-
thermoplastics
No cross links between chains. The lack of cross links allows recycling of polymers by heating it above the glass transition material,
T_g, lowering the viscosity.An example of thermoplastics is PET, used in water bottles
-
thermosets
has lots of cross-links between chains, making it more rigid. Heating does not lower its viscosity making them much harder/impossible to recycle.
and example of thermosets is melamine formaldehyde, used on kitchen tabletops
-
elastomers
has some cross links and a lot of folding of chains
Latex is an example of an elastomer
Polymers are relatively new materials, lightweight, durable, flammable, and degraded by UV light. They are made of long carbon-carbon chains.
Stress-Strain Curve of Polymers
Thermoplastics
The simplest polymer is poly(ethene):
When 2 polymer chains get close together, Van der Waals (vdw) forces keep them together. vdw forces are very weak, much weaker than the covalent bonds inside the polymer.
Stress Strain Curve
- During linear deformation, the carbon chains are strethed.
- At yield stress, the carbon chains get untangled and slide past eache other.
- Necking initially allows the chains to slide at lower stress.
- As the chains pull, align, and get closer, the vdw forces get stronger and more stress is required to fracture.
Crystalline and Amorphous/Glassy Solids (Heating and Cooling)
Amorphous Thermoplastics
- As you heat above
T_g, the chains get easier to move past each other. - It is known as an amorphous supercooled liquid.
- There is not really a melting point are there are no crystals, but
T_mis the point where the chains are easy to move
Crystalline Polymers
- The glass transition point does not exist for crystalline polymers
- The solid is difficult to deform below
T_mand is not ductile - Above
T_mthe chains are very easy to move past each other
Semi-Crystalline
- Below
T_g, only local movements in chains are possible, so the material is less ductile. The solid crystalline regions makes it difficult to move the chains. - Between
T_gandT_m, the glassy chains are easier to move but the crystalline regions remain difficult - Above
T_mthe chains easily move past each other
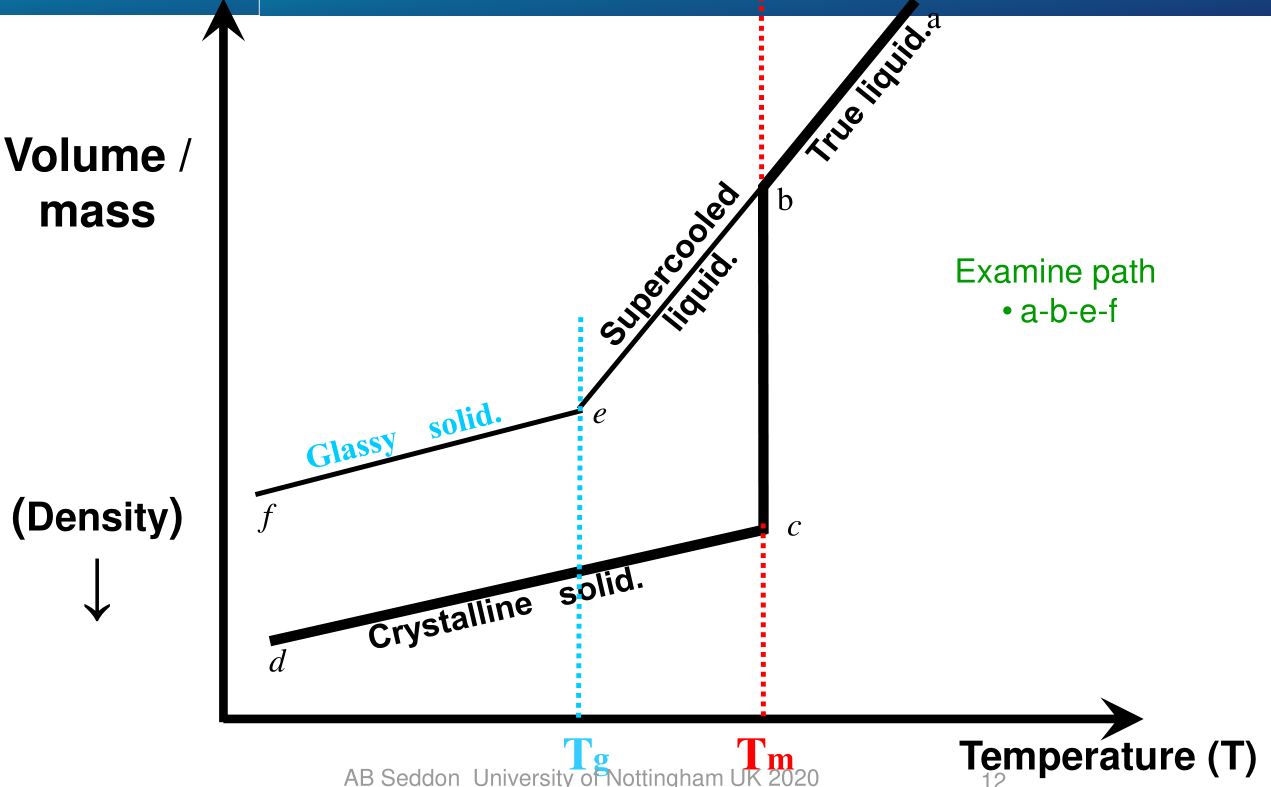
Specific Volume vs Temperature
Path ABCD
- a-b --- Start cooling the true liquid
- b-c --- At the freezing point,
T_m, the true liquid freezes diretly to a crystalline solid - c-d --- The crystalline solid cools t room temperature as the temperature is lowered
Path ABEF
-
a-b --- start cooling the true liquid
-
b --- at the freezing point nothing freezes
-
b-e --- the liquid becomes supercooled and contracts and becomes more viscous as the temperature decreases.
The supercooled liquid region is between
T_gandT_mSupercooling requires you to cool the sample quicker than you would for path ABCD
-
e ---
T_gis reached and the supercooled liquid sets to a amorphous solid -
e-f --- the amorphous solid cools from room temperature and contract as the temperature is lowered
Relative Molar Mass and Degree of Polymerisation
- Number Average RMM ---
\bar M_n = \sum x_iM_i - Weight Average RMM ---
\bar M_w = \sum w_iM_i - Degree of polymerisation ---
n_n = \frac {\bar M_n} mandn_w = \frac {\bar M_w} m
where
M_iis the RMM of the chainx_iis the fraction of the polymer that is composed of that chain by number/quantityw_iis the fraction of the polymer that is composed of that chain by mass/weightmis the RMM of the monomer from which the polymer was made
Making Polymers
There are two ways to make polymers:
Elastic Deformaion
Elastic deformation is deformation where the material will return to original shape after the applied stresses are removed.
Elastic deformation is the first type of deformation that happens when stresses are applied to a material and is represented by the straight line at the beginning of a stress-strain curve.
Modulus of Resillience (E_r)
This is the area under the elastic portion of a stress-strain graph of a material.
Plastic Deformation
Toughness (Absorbing Energy Through Plastic Deformation)
- The toughness of a material is its ability to absorb energy through plastic deformation without fracturing
- The material toughness of a ductile material can be determined by finding the area under its stress-strain curve (e.g. by integrating the graph)
- Brittle materials like ceramics and glasses exhibit no material toughness
- Ductile materials have a possibility of achieving large material toughness
Ductility measures how much something deforms plastically before fracture, but just because a material is ductile does not make it tough.
The key to high material toughness is a good combination of large ultimate fracture stress and large ductility.
-
The unit of toughness is energy per unit volume as toughness can be mathematically expressed as:
toughness = \int^{\varepsilon_f}_0\! \sigma \,\mathrm{d}\varepsilon = \frac{\text{Energy}}{\text{Volume}} $$ -
A metal may have satisfactory toughness under static loads but fail under dynamic loads or impact
This may be caused by the fact that ductility and toughness usually decrease as rate of loading increases.
-
Ductility and toughness decreasee with temperature
-
Notches in the material affect the distribution of stress in the material, potentially changing it from a uniaxial stress to multiaxial stress
Charpy Impact Test
Measures material toughness by determining the amount of energy absorbed during fracture.
It works by essentially dropping a hammer into a sample whose dimensions are standardized (usually either by BSI or ISO) and measuring how high the hammer goes up on the other side, after it breaks the material
The height of the hammer after impact will tell you how much enery is left in it, and therefore how much has been aborbed by the now broken sample.
Under a microscope, more ductile fractures appear fibrous or dull, whereas less ductile surfaces have granular or shiny surface texture.A
The charpy test has a couple issues:
- Results are prone to scatter as it is difficult to achieve a perfectly shaped notch
- Temperature has to be strictly controlled since it affects a material's ductility
The setup of a charpy impact test
- Sample is made to standardized dimensions, with a notch
- Sample is placed on support
- A very heavy hammer pendulum of mass
mis dropped from rest ath_0to swing about a pivot, reachingE_{kmax}vertically below the pivot.
a. If no sample is in place then the hammer will swing back up on the other side to a height of
h_h where theoretically $h_h = h_0$
b. With a sample placed vertically below, some of the E_k is transferred to the sample to bend
and (usually) break the sample.
If breaks the sample, it will swing up to the other side, where its max height, $h_f$ can be
used to calculate how much energy was used to break the sample:
$$E = mg(h_h-h_f)$$
Where $g$ is acceleration due to gravity.
Ductility
Ductility is the plastic deformation a material withstands before fracture.
Griffith Surface Flaws
These flaws vary in size and shape. They limit the ability of any material, brittle or ductile, to withstand tensile stresses as they concentrate the tensile forces applied to a smaller area.
The stress at the tip of the flaw:
\sigma_{actual} = 2\sigma\sqrt{\frac a r}For deep (a is large) or thin (r is small) the stress is magnified and, if it exceeds the UFS
in a brittle material, the flaw will grow into a crack, resulting in the brittle material
fracturing.
However in a ductile material, the tip of the flaw can heal, reducing a and increasing r.
This is due to the chemical structure of ductile materials like metals.
Stress Intensity Factor
Stress Intesity Factor, K:
K = f\sigma\sqrt{\pi a}where:
fis the geometry factor (1 would represent an infinite width sample, and 0 a 0 width sample)\sigmais applied tensile strengthais flaw depth
Fracture Toughness
The value of K that causes the notch to grow and cause fractures.
This is value is known as the fracture toughness, K_c.
At low thicknesses fracture toughness depends on thickness but as thickness increases, $K_c$
decreases to the constant value, the plane strain fracture toughness, K_{1c}.







![An example sample for testing fracture toughenss. From: [accessed 8 Nov, 2021]](/alvierahman90/notes/media/commit/ce582bbee3dc89a4c264cdea05b4148705836da8/mechanical/images/Compact-tension-sample-geometry-used-for-fracture-toughness-measurement.png)